 Why use Humic Substances for Carbon Sequestration
Why use Humic Substances for Carbon Sequestration
 Definition of Humic Substances
Definition of Humic Substances
Humic substances (HSs) are naturally occurring brown and black plant organic matter. They are comprised of humic acid, fulvic acid, and humin, which together is commonly referred as “Humus” Importance of humus in agricultural, has been known for a long time. Today, they can play a major role in sequestering carbon in soils and by carbon dioxide uptake by increased biomass and thus averting the adverse consequences of climate changes due to excessive building up of carbon dioxide by increased burning of carbon fuels.
Humic substances not only the largest component of soil organic matter but also account for as much as 95% of the total dissolved organic matter in natural waters and they often exceed the concentrations of ions like calcium, sodium and chloride ions present. HSs act as buffers and they help to counteract the adverse effects of acid rain on lakes, rivers and forests. Most importantly, humic substances (which account for 50 - 80% of all soil organic matter) are a sizeable part of the enormous carbon pool on our planet, as shown in the following Table 1. Described below are the benefits of converting plant and animal waste and coal to give long-lived humic substances. This is nature’s way of preserving the carbon balance in the environment as well as producing materials with remarkable life-sustaining properties.
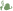 Humic Substances Properties
Humic Substances Properties
The America Society of Agronomy classifies the three components of HS s based on solubility. Fulvic acids (yellow) are very soluble in water, but brown humic acids (HAs) precipitate when acid is added to HA solutions. Humins (with a brown-black color) are insoluble in water and are believed to consist of humic acid molecules firmly attached to clay (such as kaolinite) or a mineral (such as hematite).
Humic acids (HAs) are the major fraction of humic substances. They contain more carbon than all living things. HAs exist in plants, soils, water, municipal solid waste, compost and sewage, and they can be isolated from soft coals. HAs strongly retain water and they are the buffer and matrix of many chemical and biochemical reactions in soils.
 Humic Acid Structures and Reactions
Humic Acid Structures and Reactions
Although the detailed molecular structures of HAs are unknown at present, HAs behaved as if they have two main structural features. One feature is chemical functional groups, which help HAs to direct physical and chemical reactions. Chemical functional groups present are: carboxylic, phenolic, aliphatic and enolic-OH and carbonyl (C=O). Example of a physical reaction is the binding of a plant nutrient metal such as magnesium (thus concentrating the metal for absorption through the plant roots). Another is binding of a toxic metal such as cadmium (tying up the metal and keeping it out of the water supply). Another physical reaction is retention of water, which is essential for plant growth.
HA’s also direct chemical reactions because they can accept and donate electrons. For example, HA’s can convert carcinogenic chromate in the water from plating and tanning factories to non-toxic chromic ions. A biochemical example is that HAs act as mediators in the reactions of microbes with iron-containing minerals. The HAs help to transfer electrons produced by the microbes to the minerals as the microbes eat and grow. The microbes themselves cannot interact directly with the minerals, but HAs can.
 How Do Humic Acids Remain Stable?
How Do Humic Acids Remain Stable?
Chemical or biological mineralization and thermal oxidation of any organic material releases carbon dioxide in the air. If all the carbon behaved in this way we would have only plants (grown by photosynthesis) on the land and carbon dioxide in the air, but there would be no organic matter in the soils. (Some carbon dioxide in the air interacts with inorganic compounds such as limestone on the land and in water sediments, and this helps to somewhat reduce the amount of CO2 in the air). Plants need water, air and soil to grow and reproduce. The fact is that soils exist because humic substances are stable compared to carbohydrates and other plant and animal molecules. Carbon dating indicates that some HSs are 10,000 to 30,000 years old.
We noted above that HSs have functional groups that they use to bind metals and retain water. Their other structural feature is carbon-rich units (“greasy blobs”) that give the HA molecules some hydrophobic (water-repellant) character. One HA model has the hydrophilic (water-loving) functional groups on the outside and the hydrophobic groups on the inside of a molecule that looks like a telephone cord. The molecule presumably can “turn itself inside out” as necessary, making the outside hydrophobic.
The scanning electron micrograph below shows a sample of a solid HA and the Figure shows a proposed molecular structure that has a mainly hydrophilic exterior.
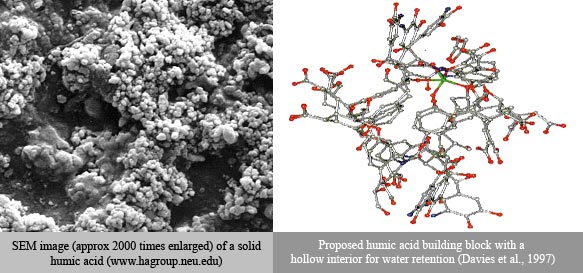
Another way for HAs to resist mineralization is by attaching themselves to minerals using their functional groups. This makes the surface of the mineral “greasy” and leaves the HAs less susceptible to oxidation. A third possibility is that solid HAs have pores that are too small for microbes to pass through, hiding the carbon the microbes need for food.
Research shows that biomass composting and humification proceed in stages of increasing time span. Depending on the biomass source, moisture content, the microenvironment and other factors, the humification stages in the first 60-70 days are exothermic and may result in up to 50% carbon mineralization of the most active components, as monitored by the atomic C/N ratio, which is a measure of humus stability. During humification, the ratio NO3-/NH4+ increases to about 4 and is an index of humified compost maturity.1
A study of municipal solid waste (MSW) composting showed that the total quantity of humic acid (HA) was steady during the several stages of humification and maturation that involve mineralization of oxidatively unstable MSW components.1 This is significant since
- HA is the major humic component of every productive soil [the MSW sample1 was already partly composted]
- HA is the most chemically stable organic soil component
Studies based on radiochemical 14C dating have shown that HAs in Andosols, Chernozems and other soils were formed several thousand years ago and survive to this day.2-15 Attachment to minerals stabilizes HAs11,13 and it is significant that coal-derived HAs have a high mineral content (a typical ash content of lignite-derived HA is 34%w/w) 34%w/w)16. This is significant because HAs from ancient coal sources are not only stable but also being producted as highly stable cross-linked humic material for water clean up.
The resultant HAs are dispersed in and on soil to improve its ability to:
- Retain water
- Exist as hydrogels [converted by gel processing to give aerogels]
- Buffer soil pH
- Enhance soil structure and texture
- Bind fertilizer and inhibit soil runoff
- Sequester heavy metals
- Catalyze redox reactions
- Sequester and transform PAHs and PCBs in contaminated soils
- Bind RNA and DNA (life perhaps began on surfaces)
- Simultaneously remove TCE, PCE, other POCs and metals from contaminated water at civil, industrial and military facilities
- Prevent beach and soil erosion by binding to minerals, and enable creation of new soil from the desert
 HAs for Soil & Water Treatment, Recycling & Carbon Sequestration
HAs for Soil & Water Treatment, Recycling & Carbon Sequestration
Plants and coals are the products of photosynthesis. The carbon they contain once was CO2 in the air. Burning plant
bio-waste or coal returns the CO2 to the air and defeats attempts at carbon sequestration as a remedy for global warming.
On the other hand, conversion of bio-waste and low rank coal to HAs retains the carbon they contain while at the same time providing materials with truly remarkable properties.
Plants need air, water, and soil to grow, and the soil they need contains HAs that:
- Create and maintain soil structure
- Retain water
- Bind and release plant nutrients
- Sequester toxic metals and other contaminants
- Serve as a matix for soil chemical and biochemical reaction
Because they are multifunctional materials, synthetic solid HAs have the ability to remove both inorganic and organic contaminants from wastewater. This approach is being used to decontaminate and recycle wastewater for agricultural use in several countries where the only alternative is a lifeless drought.
Bio-waste composting and conversion of coals to humic substances keeps the carbon produced by photosynthesis on the land instead of as a greenhouse gas in the air. A sensible approach to climate control is an increased, worldwide emphasis on composting and coal conversion in parallel with the development of alternative sources of energy.
 How HA Production from Bio-Waste and Coals Helps in Repairing Impaired Lands and Arid Areas While Sequestering Carbon
How HA Production from Bio-Waste and Coals Helps in Repairing Impaired Lands and Arid Areas While Sequestering Carbon
Plant bio-waste is increasingly being used as a source of alternative fuels (ethanol, bio-diesel), but this is no solution to global warming. Converting that biomass to humic substances through compositing sequesters carbon while at the same time yielding long-lived humic materials with a host of useful properties. Another way to sequester carbon is not to burn low-fuel-value soft coals but instead to convert them to humic substances for application on impaired lands and arid areas, recovering them for habitation and agriculture.
 How to Calculate Carbon Sequestration Potential from Applications of Humic Substances.
How to Calculate Carbon Sequestration Potential from Applications of Humic Substances.
Increased carbon sequestration from humic acid addition to soils will be gradual, but most sequestration will result from increased biomass growth stimulated by use of humic products in impaired soils which otherwise are not amenable to biomass cultivation. For example, about 6 tons/yr of CO2 is captured on each acre of land with tree biomass planted only on 10% of land, which can be intercropped with food crops on the remaining 90%. The Kyoto Treaty qualifies this reduction for a 70-years average active growth cycle of trees via the provisions of clean development mechanisms. Other approaches still under consideration indicate acceptance of this approach for 100 years or more, since trees can sequester CO2 for centuries.
 References
References
- Chen, Y.; Chefetz, B.; Adani, F.; Genevini, P.; Hadar, Y. Organic matter transformation during composting of municipal soild waste. In: The Role of Humic Substances in the Ecosytems and in Environmental Protection. Drozd, J.; Gonet, S. S.; Senesi, N.; Weber, J. (eds.). Wroclaw: Polish Society of Humic Substances, 1997, p. 795-804. See also Filip, Z. K., ibid. p 805-810.
- Arslanov, K. A.; Gerasimov, I. P.; Zubkov, A. I.; Kozyreva, M. G.; Rubilin, E. V.; Chichagova, O. A. Determination of the age of chernozem using a radiocarbon method. Doklady Akademii Nauk SSSR 192: 1141-4 (1970).
- Gerasimov, I. P.; Chichagova, O. A. Radiocarbon dating of soil humus.Pochvovedenie 10: 3-11 (1971).
- Nemecek, J. Carbon-14 determination of the age of organic substances in chernozems.Rostlinna Vyroba 17: 745-51 (1971).
- Tolchel'nikov, Yu. S.; Kostarev, A. S. Radiocarbon determination of the age of alpha-humus podzol.Izv. Vses. Geogr. Ova III: 264-8 (1979).
- Matthews, J. A. Natural carbon-14 age /depth gradient in a buried soil.Naturwiss. 68: 472-4 (1981).
- Schoute, J. F. T.; Griede, J. W.; Mook, W. G.; Roeleveld, W. Radiocarbon dating of vegetation horizons, illustrated by an example from the Holocene coastal plain in the northern Netherlands.Geologie en Mijnbouw 60: 453-9 (1981).
- Schoute, J. F. T.; Mook, W. G.; Streurman, H. J. Radiocarbon dating of vegetation horizons: methods and preliminary results.PACT ( Athens, Greece) 8: 295-311 (1983).
- Chichagova, O. A.; Cherkinskii, A.; Tolchelnikov, Y. S.; Inversions of the radiocarbon age of humus in profiles of contemporary soils. Doklady Akademii Nauk SSSR 275: 1176-86 (1984).
- Yamada, Y. The characterization of humus accumulation in Andosols by carbon-14 dating.Nogyo Kankyo Gijutsu Kenkyusho Hokoku 3: 23-86 (1986).
- Theng, B. K. G.; Tate, K. R.; Becker-Heidmann, P. Establishing the age, location, and identity of the inert soil organic matter of a Spodosol.Zeit. Pflanz. Boden. 155: 181-4 (1992).
- Murphy, E. M.; Schramke, J. A.; Fredrickson, J. K.; Bledsoe, H. W.; Francis, A. J.; Sklarew, D. S.; Linehan, J. C. The influence of microbial activity and sedimentary organic carbon on the isotope geochemistry of the Middendorf aquifer.Water Resources Research 28: 723-40 (1992).
- Skjemstad, J. O.; Janik, L. J.; Head, M. J.; McClure, S. G. High-energy ultraviolet photooxidation: a novel technique for studying physically protected organic matter in clay- and silt-sized aggregates.J. Soil Sci. 44: 485-99 (1993).
- Tsutsuki, K.; Kondo, R.; Shiraishi, H.; Kuwatsuka, S. Composition of lignin-degradation products, lipids, and opal phytoliths in a peat profile accumulated since 32,000 years B.P. in central Japan.Soil Sci. Plant Nutrit., 39: 463-74 (1993).
- Cherkinsky, A. E.; Brovkin, V. A. D ynamics of radiocarbon in soils.Radiocarbon 35: 363-7 (1993). See also Baldock, J. A.; Nelson, P. N. Soil organic matter. In: Handbook of Soil Science, Sumner, M. E. (ed.). Boca Raton: CRC Press, 1999, p. B-41.
- Schnitzer, M.; Dinel, H.; Pare, T.; Schulten, H. –R.; Ozdoba, D. Some chemical and spectroscopic characteristics of six organic ores. In: Humic Substances: Structures, Models and Functions. Ghabbour, E. A.; Davies, G. (eds.) Cambridge: Royal Society of Chemistry, 2001, p. 315-328.
- Alvarez, R.; Alconada, M.; Lavada, R. Sewage sludge effects on carbon dioxide: Carbon production from a desurfaced soil. Commun. Soil Sci. Plant Anal., 30: 1861-1866 (1999).
- Fortun, C.; Fortun, A.; Almendros, G. The effect of organic materials and their humified fractions on the formation and stabilization of soil aggregates. Sci. Total Environ. 81/82: 561-568 (1989).
- Lal, R. Offsetting China’s CO 2 emissions by soil carbon sequestration. Climatic Change 65: 263-275 (2004).
- Grant, B; Smith, W. N.; Desjardins, R.; Lemke, R.; Li, C. Estimated N 2O and CO 2 emissions as influenced by agricultural practices in Canada. Climatic Change 65: 315-332 (2004).
- Nabuurs, G. J.; Mohren, G. M. J. Modelling analysis of potential carbon sequestration in selected forest types. Can J. Forest Res., 25: 1157-1172 (1995).
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Current Sci. 82: 510-517 (2002).
- Paustian, K.; Six, J.; Elliott, E. T.; Hunt, H. W. Management options for reducing CO 2 emissions from agricultural soils. Biogeochem. 48: 147-163 (2000).
- http://soils.usda.gov/sqi/concepts/soil_biology/soil_food_web.html (accessed October 5, 2007).
 Bibliography
Bibliography
- Bruccoleri, A. G., B. T. Sorenson and C. H. Langford. 2001. Molecular modeling of humic structures. In: Humic Substances: Structure, Models and Functions, Royal Society of Chemistry, Cambridge, p. 193-208.
- Chang, S. and R. A. Berner. 1998. Humic substances formation via the oxidative weathering of coal. Environ. Sci. Technol ., 32, 3883-6.
- Clapp, C. E. and M. H. B. Hayes. 1999. Sizes and shapes of humic substances. Soil Sci., 164, 777-789.
- Conte, P. and A. Piccolo. 1999. Conformational arrangement of dissolved humic substances. Influence of solution composition on association of humic molecules. Environ. Sci. Technol.,33, 1682-1690.
- Davies, G. and E. A. Ghabbour, Eds., 1998. Humic Substances: Structures, Properties and Uses. Royal Society of Chemistry, Cambridge.
- Davies, G., A. Fataftah, A. Cherkasskiy, E. A. Ghabbour, A. Radwan, S. A. Jansen, S. Kolla, M. D. Paciolla, L. T. Sein, Jr., W. Buermann, M. Balasubramanian, J. Budnick and B. Xing. 1997. Tight metal binding by humic acids and its role in biomineralisation. J. Chem. Soc. Dalton Trans. 4047-4060.
- Davies, G., E. A.Ghabbour, and C. .Steelink, 2001. Humic substances: Marvelous products of soil chemistry. J. Chem. Educ., 78, 1609-1614.
- Engebretson, R. R. and R. von Wandruszka. 1998. Kinetic aspects of cation-enhanced aggregation in aqueous humic acids. Environ. Sci. Technol ., 32, 488-93.
- Gaffney, J. S., N. A. Marley and S. B. Clark. 1996. Humic and fulvic acids and organic colloidal materials in the environment. In: Humic and Fulvic Acids: Isolation, Structure and Environmental Role, Gaffney, J. S., N. A. Marley and S. B. Clark, Eds. American Chemical Society Symposium Series 651, p. 2.
- Ghabbour, E. A., G. Davies, K. O’Donaughy, T. L. Smith and M. E. Goodwillie. 2004. Thermodynamics of humic acids adsorption on kaolinite. Environ. Sci. Technol., 38, 3338-3342. (2004).
- Hayes, M. H. B. 1998. Humic substances: Progress towards more realistic concepts of structures. In: Humic Substances: Structures, Properties and Uses, Davies, G. and E. A. Ghabbour, Eds. Royal Society of Chemistry, Cambridge, p. 1.
- Hayes, M. H. B. and W. S. Wilson, Eds., 1997. Humic Substances, Peats and Sludges: Health and Environmental Aspects, Royal Society of Chemistry, Cambridge.
- Hester, R. E. and R. M. Harrison, Eds. 1997. Issues in Environmental Science and Technology, 7: Contaminated Land and its Reclamation. Royal Society of Chemistry, Cambridge.
- Huang, P. M., D. L. Sparks and S. A. Boyd, Eds. 1998. Future Prospects for Soil Chemistry. Soil Science Society of America, Madison, Wisconsin, 1998.
- Khairy, A. H., H. H. Baghdadi and E. A. Ghabbour. 1990. Adsorption mechanisms of nicotine on humic acid and a clay humic acid complex. Z. Pflanzenernahr. Bodenk ., 153, 33-38.
- Kinniburgh, D.G., C. J. Milne, M. F. Benedetti, J. P. Pinheiro, J. Filius, L. K. Koopal and W. H. van Riemsdijk. 1996. Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. , 30, 1687-1698.
- Klavins, M. 1998. Aquatic humic substances. Lu, Riga.
- Lovley, D. R., J. L. Fraga, E. L. Blunt-Harris, L. A. Hayes, E. J. P. Phillips and J. D. Coates. 1998. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol.,26, 152-157.
- MacCarthy, P. and J. A. Rice. 1994. Industrial applications of humus: An overview. In: Humic substances in the global Environment and implications on human health, Senesi, N. and T. M. Miano, Eds. Elsevier, Amsterdam. p. 1209-1223.
- MacCarthy, P., C. E. Clapp, R. L. Malcolm and P. R. Bloom, Eds. 1990. Humic Substances in Soil and Crop Science: Selected Readings. American Society of Agronomy, Madison, Wisconsin.
- Mao, J., W.-G. Hu, K. Schmidt-Rohr, G. Davies, E. A. Ghabbour and B. Xing. 2000. Quantitative characterization of humic acids by solid-state 13C NMR. Soil Sci. Amer. J., 64, 873-884.
- McBride, M. B. 1994. Environmental chemistry of soils. Oxford University Press, Oxford. Chs. 2, 9 and 10.
- Murphy, E. M., J. M. Zachara and S. C. Smith. 1994. Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ. Sci. Technol.,28, 1291-9.
- Perdue, E. M. 2000. Modeling concepts of metal-humic complexation. In: Humic Substances and Chemical Contaminants, Clapp, C. E., M. H. B. Hayes, N. Senesi, P. R. Bloom and P. M. Jardine, Eds. Soil Sciences Society of America, Madison, WI, Chapter 16.
- Piccolo, A., S. Nardi and G. Concheri. 1996. Micelle-like conformation of humic substances as revealed by size exclusion chromatography. Chemosphere, 33, 595.
- Ricca, G., C. Pastorelli and F. Severini. 1997. Humic acid from Leonardite: Structural investigations and complexes with metal ions. In: The Role of Humic Substances in the Ecosystems and in Environmental Protection, Drozd, J., S. S. Gonet, N. Senesi and J. Weber, Eds., Polish Society of Humic Substances, Wroclaw, Poland, p. 175-181.
- Rice, J. A. and P. MacCarthy. 1989. Characterization of a stream sediment humin. In: Influence of Aquatic Humic Substances on Fate and Treatment of Pollutants, Suffet, I. H. and P. MacCarthy, Eds. ACS Adv. Chem. Ser., No. 219, American Chemical Society, Washingion, D. C., p. 41-54.
- Sbardellati, R., F. Crescenzi and A. Robertiello. 1994. The adsorption of lead (II) by humic products from coal: An industrial application. In: Humic Substances in the Global Evironment and Implications on Human Health, Senesi, N. and T. M. Miano, Eds. Elsevier, Amsterdam. p. 1353-1358.
- Schulten, H. R. and M. Schnitzer. 1995. Three-dimensional models for humic acids and soil organic matter. Naturwiss. 82, 487-498.
- Scott, D. T., D. M. McKnight, E. L. Blunt-Harris, S. E. Kolesar and D. R. Lovley. 1998. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol.,32, 2984-2989.
- Senesi, N. 1994. Spectroscopic studies of metal ion-humic substance complexation in soil. Trans. World Congress Soil Sci., 15th. 3a: 384-402.
- Senesi, N. and T. M. Miano, Eds. 1994. Humic substances in the global environment and implications on human health. Elsevier, Amsterdam.
- Senesi, N., G. Brunetti, E. Loffredo and T. M. Miano. 1999. Abiotic catalytic humification of organic matter in olive oil wastewaters. In: Understanding Humic Substances: Advanced Methods, Properties and Applications, Ghabbour, E. A. and G. Davies, Eds. Royal Society of Chemistry, Cambridge, p. 9-19.
- Senesi, N., P. La-Cava and T. M. Miano. 1997. Adsorption of imazethapyr to amended and non-amended soils and humic acids. J. Environ. Quality , 26, 1264-70.
- Sparks, D. L. 1995. Environmental Soil Chemistry . Academic Press, San Diego.
- Stevenson, F. J. 1994. Humus Chemistry, 2nd. Edn. New York: Wiley.
- Stevenson, F. J. 1999. Cycles of Soil. Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients . 2 nd Edn., Wiley, New York.
- Stevenson, F. J. and M. S. Ardakani. 1972. Organic matter reactions involving micronutrients in soils, In: Micronutrients in Agriculture, Mortvedt J. J., et al., Eds. Soil Science Society of America. Madison, WI, p. 79-114.
- Wershaw, R. L. and Goljer, I. 2001. Use of 13C NMR and FTIR for elucidation of degradation pathways during natural litter decomposition and composting. IV. Characterization of Humic and Fulvic Acids Extracted from Senescent Leaves. In: Humic Substances : Structures, Properties and Uses , Davies, G. and E. A. Ghabbour, Eds. Royal Society of Chemistry, Cambridge, p. 61-68, and references therein.
- Xing, B., J. Mao, W. -G. Hu, K. Schmidt-Rohr, G. Davies and E. A. Ghabbour. 1999. Evaluation of different solid-state 13C NMR techniques for characterizing humic acids. In: Understanding Humic Substances: Advanced Methods, Properties and Applications, Ghabbour, E. A. and G. Davies, Eds. Royal Society of Chemistry, Cambridge, p. 49-63.
- Yates, L. M., III and R. von Wandruska. 1999. Decontamination of polluted water by treatment with a crude humic acid blend, Environ. Sci. Technol ., 33, 2076-80.
- Zhang, Y. J., N. D. Bryan, F. R. Livens and M. N. Jones. 1996. Complexing of metal ions by humic substances. In: Humic and Fulvic Acids: Isolation, Structure and Environmental Role, Gaffney, J. S., N. A. Marley and S. B. Clark, Eds. American Chemical Society Symposium Series 651, p. 194.
 Supplemental Reading
Supplemental Reading
-
Bertoldi, M. et al (Eds.). The Science of Composting: European Commission International Symposium. London: Blackie, 1996, Volumes 1 and 2.
-
Bruccoleri, A. G., B. T. Sorenson and C. H. Langford. 2001. Molecular modeling of humic structures. In: Humic Substances: Structure, Models and Functions, Ghabbour E. A. and G. Davies (Eds.), Royal Society of Chemistry, Cambridge, p. 193-208.
-
Chang, S. and R. A. Berner. 1998. Humic substances formation via the oxidative weathering of coal. Environ. Sci. Technol ., 32: 3883-6.
-
Clapp, C. E. and M. H. B. Hayes. 1999. Sizes and shapes of humic substances. Soil Sci., 164: 777-789.
-
Conte, P. and A. Piccolo. 1999. Conformational arrangement of dissolved humic substances. Influence of solution composition on association of humic molecules. Environ. Sci. Technol., 33: 1682-1690.
-
Davies, G., A. Fataftah, A. Cherkasskiy, E. A. Ghabbour, A. Radwan, S. A. Jansen, S. Kolla, M. D. Paciolla, L. T. Sein, Jr., W. Buermann, M. Balasubramanian, J. Budnick and B. Xing. 1997. Tight metal binding by humic acids and its role in biomineralisation. J. Chem. Soc. Dalton Trans. 4047-4060.
-
Davies, G., E. A. Ghabbour, and C. Steelink, 2001. Humic substances: Marvelous products of soil chemistry. J. Chem. Educ ., 78: 1609-1614.
-
Engebretson, R. R. and R. von Wandruszka. 1998. Kinetic aspects of cation-enhanced aggregation in aqueous humic acids. Environ. Sci. Technol ., 32: 488-93.
-
Gaffney, J. S., N. A. Marley and S. B. Clark. 1996. Humic and fulvic acids and organic colloidal materials in the environment. In: Humic and Fulvic Acids: Isolation, Structure and Environmental Role, Gaffney, J. S., N. A. Marley and S. B. Clark, Eds. American Chemical Society Symposium Series 651, p. 2.
-
Ghabbour, E. A., G. Davies, K. O’Donaughy, T. L. Smith and M. E. Goodwillie. 2004. Thermodynamics of humic acids adsorption on kaolinite. Environ. Sci. Technol., 38: 3338-3342.
-
Hatcher, P. G., I. A. Breger, L. W. Dennis and G. E. Maciel. 1982. Chemical structures in coal: NMR studies and a geochemical approach: American Chemical Society, Division of Fuel Chemistry, Preprints, 27: 172-183.
-
Hatcher, P. G., I. A. Breger, G. E. Maciel and N. M. Szeverenyi. 1983. Chemical structures in coal: Geochemical evidence for the presence of mixed structural components: 1983 International Conference on Coal Science, Proceedings, p. 310-313.
-
Hayes, M. H. B. 1998. Humic substances: Progress towards more realistic concepts of structures. In: Humic Substances: Structures, Properties and Uses, Davies, G. and E. A. Ghabbour, Eds. Royal Society of Chemistry, Cambridge, p. 1.
-
Hester, R. E. and R. M. Harrison, Eds. 1997. Issues in Environmental Science and Technology, 7: Contaminated Land and its Reclamation. Royal Society of Chemistry, Cambridge.
-
Huang, P. M., D. L. Sparks and S. A. Boyd, Eds. 1998. Future Prospects for Soil Chemistry. Soil Science Society of America, Madison, Wisconsin.
-
Kinniburgh, D.G., C. J. Milne, M. F. Benedetti, J. P. Pinheiro, J. Filius, L. K. Koopal and W. H. van Riemsdijk. 1996. Metal ion binding by humic acid: Application of the NICA-Donnan model. Environ. Sci. Technol. , 30: 1687-1698.
-
Lovley, D. R., J. L. Fraga, E. L. Blunt-Harris, L. A. Hayes, E. J. P. Phillips and J. D. Coates. 1998. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim. Hydrobiol., 26: 152-157.
-
MacCarthy, P. and J. A. Rice. 1994. Industrial applications of humus: An overview. In: Humic substances in the global Environment and implications on human health, Senesi, N. and T. M. Miano, Eds. Elsevier, Amsterdam. p. 1209-1223.
-
MacCarthy, P., C. E. Clapp, R. L. Malcolm and P. R. Bloom, Eds. 1990. Humic Substances in Soil and Crop Science: Selected Readings. American Society of Agronomy, Madison, Wisconsin.
-
Mayhew, L. Humic substances in biological agriculture. Acres, 34: (2004) – reprint available.
-
McBride, M. B. 1994. Environmental chemistry of soils. Oxford University Press, Oxford. Chs. 2, 9 and 10.
-
Murphy, E. M., J. M. Zachara and S. C. Smith. 1994. Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ. Sci. Technol., 28: 1291-9.
-
Olk, D. C., M. C. Dancel, E. Moscoso, R. R. Jimenez, and F. M.Dayrit. 2002. Accumulation of lignin residues in organic matter fractions of lowland rice soils: a pyrolysis-GC-MS study.Soil Science. 167(9): 590-606.
-
Olk, D. C. and E. G.Gregorich. 2006. Overview of the symposium proceedings, "Meaningful pools in determining soil carbon and nitrogen dynamics.Soil Science Society of America Journal, 70(3): 967-974.
-
Perdue, E. M. 2000. Modeling concepts of metal-humic complexation. In: Humic Substances and Chemical Contaminants, Clapp, C. E., M. H. B. Hayes, N. Senesi, P. R. Bloom and P. M. Jardine, Eds. Soil Sciences Society of America, Madison, WI, Chapter 16.
-
Ricca, G., C. Pastorelli and F. Severini. 1997. Humic acid from Leonardite: Structural investigations and complexes with metal ions. In: The Role of Humic Substances in the Ecosystems and in Environmental Protection, Drozd, J., S. S. Gonet, N. Senesi and J. Weber, Eds., Polish Society of Humic Substances, Wroclaw, Poland, p. 175-181.
-
Rice, J. A. and P. MacCarthy. 1989. Characterization of a stream sediment humin. In: Influence of Aquatic Humic Substances on Fate and Treatment of Pollutants, Suffet, I. H. and P. MacCarthy, Eds. ACS Adv. Chem. Ser., No. 219, American Chemical Society, Washington, D. C., p. 41-54.
-
Sbardellati, R., F. Crescenzi and A. Robertiello. 1994. The adsorption of lead(II) by humic products from coal: An industrial application. In: Humic Substances in the Global Evironment and Implications on Human Health, Senesi, N. and T. M. Miano, Eds. Elsevier, Amsterdam. p. 1353-1358.
-
Schulten, H. R. and M. Schnitzer. 1995. Three-dimensional models for humic acids and soil organic matter. Naturwiss. 82: 487-498.
-
Senesi, N. 1994. Spectroscopic studies of metal ion-humic substance complexation in soil. Trans. World Congress Soil Sci., 15th. 3a: 384-402.
-
Senesi, N., G. Brunetti, E. Loffredo and T. M. Miano. 1999. Abiotic catalytic humification of organic matter in olive oil wastewaters. In: Understanding Humic Substances: Advanced Methods, Properties and Applications, Ghabbour, E. A. and G. Davies, Eds. Royal Society of Chemistry, Cambridge, p. 9-19.
-
Senesi, N., P. La-Cava and T. M. Miano. 1997. Adsorption of imazethapyr to amended and non-amended soils and humic acids. J. Environ. Quality , 26: 1264-70.
-
Stevenson, F. J. 1994. Humus Chemistry, 2nd. Edn. New York: Wiley.
-
Stevenson, F. J. 1999. Cycles of Soil. Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients . 2 nd Edn., Wiley, New York.
-
Wershaw, R. L. and Goljer, I. 2001. Use of 13C NMR and FTIR for elucidation of degradation pathways during natural litter decomposition and composting. IV. Characterization of humic and fulvic acids extracted from senescent leaves. In: Humic Substances: Structures, Properties and Uses, Davies, G. and E. A. Ghabbour, Eds. Royal Society of Chemistry, Cambridge, p. 61-68, and references therein.
-
Yates, L. M., III and R. von Wandruska. 1999. Decontamination of polluted water by treatment with a crude humic acid blend, Environ. Sci. Technol., 33: 2076-80.
-
Zhang, Y. J., N. D. Bryan, F. R. Livens and M. N. Jones. 1996. Complexing of metal ions by humic substances. In: Humic and Fulvic Acids: Isolation, Structure and Environmental Role, Gaffney, J. S., N. A. Marley and S. B. Clark, Eds. American Chemical Society Symposium Series 651, p. 194.